Drug Development
Usona’s drug development efforts continued to make strong, measurable progress this year, reflecting our long-standing commitment to quality, rigorous science, and cross-functional collaboration. Across both our psilocybin and 5-MeO-DMT programs, we advanced key regulatory, clinical, and Chemistry, Manufacturing, and Controls (CMC) milestones that prepare us for successful late-stage development and future patient access. Our work remains focused on moving safe, effective, and evidence-based treatments through the regulatory process efficiently and responsibly, so that care can reach those who need it most.
Usona currently has two investigational molecules in clinical development:
Psilocybin Program
PSIL301 Phase 3:
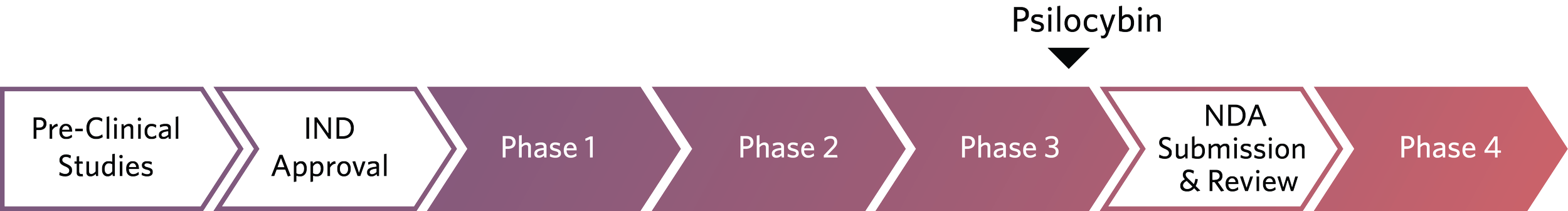
Trial for Major Depressive Disorder
In 2025, Usona continued to execute the uAspire (PSIL301) Phase 3 study with a focus on data quality, participant safety, diversity, and regulatory alignment.
As this phase approaches completion, we are fully mobilizing toward New Drug Application (NDA) submission and preparing the systems necessary to support safe and accessible future care delivery.
uAspire evaluates whether a single, supervised administration of psilocybin, with psychosocial support, can provide meaningful and durable symptom relief for people living with MDD.
Highlights
-
The study activated 30 clinical sites across the U.S., including 4 Veterans Affairs (VA) medical centers, broadening geographic reach and participant diversity.
-
In 2025, Usona continued productive engagement with the FDA under Breakthrough Therapy Designation:
Completed Type B meeting with the FDA, receiving guidance that informs our regulatory submission preparation
• Ongoing collaboration on clinical development strategy and submission planning
• Preparing for continued FDA discussions in 2026 as we advance toward New Drug Application (NDA) submission
-
In 2025, Usona achieved critical CMC milestones that support regulatory submission readiness and future commercial supply:
Manufacturing Validation: Completed commercial-scale validation batches, demonstrating reproducible manufacturing processes.
Manufacturing Partnerships:Active pharmaceutical ingredient (API) manufacturing at two global locations with maintained regulatory inspection readiness
Clinical drug product packaging and distribution with established quality agreements and global supply networks
Quality Systems: Comprehensive Quality Management System (QMS) infrastructure supports all GMP operations, documentation control, and regulatory compliance.
Regulatory Documentation: Advanced CMC documentation package development for NDA submission.
Stability Program: Sustained long-term stability program provides data supporting shelf-life claims.
Global Supply Chain: Multi-continent manufacturing capabilities established to support resilient supply and international research collaborations. -
Completed the necessary pre-clinical and toxicology requirements in preparation for FDA submission.
-
Over 130 facilitators trained across all 30 Phase 3 sites to ensure consistency in patient care, long term follow up, and patient education.
-
Guided by a Diversity Steering Committee, the plan incorporated insights from community outreach and diversity experts to recruit underrepresented populations and foster a wider enrollment in the PSIL301 Phase 3 study.
ENROLLMENT MILESTONE
In November 2025, enrollment in the uAspire (PSIL301) Phase 3 study was completed, representing a significant milestone as we advance toward regulatory submission.
As Usona nears completion of Phase 3, early work is underway to ensure responsible patient access post-approval. Teams across Regulatory, Clinical Operations, CMC, Medical Affairs, Distribution and Logistics, and Strategic Partnerships are coordinating to prepare for future delivery and patient care pathways.
Recognizing that safe and equitable access requires thoughtful preparation, Usona is developing a comprehensive delivery framework.
This cross-functional effort ensures that Usona is prepared not only for regulatory approval, but for the complex work of delivering safe, effective, and accessible care.
Preparing for Delivery of Care
Highlights
-
Designing safety protocols appropriate for psilocybin therapy delivery.
-
Engaging stakeholders including payors, health systems, federal partners (VA, DoD), and academic institutions to support responsible and affordable post-approval access.
5-MeO-DMT Program
In 2025, Usona achieved several foundational milestones that position the 5-MeO-DMT program to move into Phase 2 efficacy studies.
-
Successful GMP manufacturing of active and placebo drug product to enable Phase 2 studies.
All analytical testing and initial stability assessments confirmed quality and integrity.
Method validation and compendial testing for the active drug substance completed.
-
Finalized Chemistry, Manufacturing, and Controls documentation for inclusion in the IND.
Completed the draft MEO201 study protocol, vendor selection, and study start-up processes.
-
Developed a facilitator-training model appropriate for a Phase 2 efficacy study.
Early stability findings and import/export permit processes have been addressed to support future U.S. and international research collaborations.
Highlights